
Results with Mytesi

Results with Mytesi

Mytesi has been demonstrated to relieve symptoms of noninfectious HIV-related diarrhea in adult patients who are on antiretroviral therapy (ART).
In a clinical study known as the ADVENT trial, 374 adults living with HIV on antiretroviral therapy (ART) who had been experiencing noninfectious diarrhea for at least 1 month were given either 125 mg of Mytesi twice a day or a placebo (sugar pill).1 The study looked at how effective treatment was in an ethnically diverse patient population, comprised predominantly of male long-term survivors (LTSs).*
*LTS is a person living with HIV for >10 years.
Trial participants were:
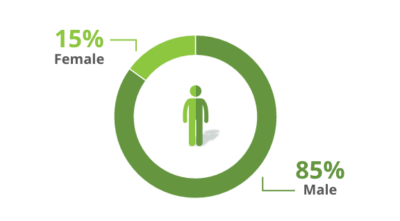
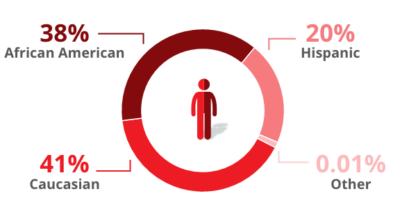
*ITT population: 125 mg BID (n=136), 250 mg BID (n=54), 500 mg BID (n=46), Placebo BID (n=138).
After 4 weeks of
Mytesi treatment2
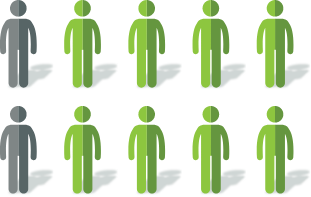
~8 out of 10 patients reported a decrease in watery stools
Among these patients, ~6 out of 10 reported at least a 50% decrease in watery stools
After 20 weeks of
Mytesi treatment2
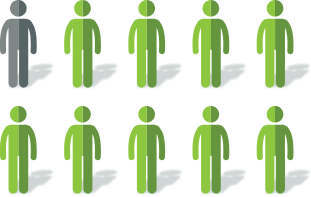
~9 out of 10 patients reported a decrease in watery stools
Among these patients, ~6 out of 10 reported no watery stools
Mytesi is well tolerated3
References: 1. Gehrig M, Clay P, Perry R, et al. Actual versus perceived use of pharmacokinetic (primarily absorption) influential OTC agents and ART tolerability in a nationwide matched cohort of HIV patients and their healthcare providers. Poster abstract presented at: ID Week 2016; October 26-30, 2016; New Orleans, LA. 2. MacArthur RD, Clay P, Blick G, et al. Long-Term Crofelemer Provides Clinically Relevant Reductions in HIV-Related Diarrhea. Poster presented at: 9th IAS Conference on HIV Science (IAS 2017); 2017 July 23-26; Paris, France. 3. MacArthur RD, Hawkins TN, Brown SJ, et al. Efficacy and safety of crofelemer for noninfectious diarrhea in HIV-seropositive individuals (ADVENT Trial): a randomized, double-blind, placebo-controlled, two-stage study. HIV Clinical Trials. 2013;14(6):261-273. doi:10.1310/hct1406-261






